For lithium-ion batteries, researchers created a new, safer electrolyte that performs just as well at low temperatures as it does at normal temperature.


Drivers of electric vehicles who reside in cold areas are aware that freezing conditions have an adverse effect on the lithium-ion batteries in their vehicles. They don’t go as far or charge as quickly. Although the issue exists, Argonne National Laboratory claims to have a potential solution.
The liquid electrolyte that acts as a conduit for ions to move between the cathode and the anode when the battery charges and discharges, according to Argonne scientists, starts to freeze at sub-zero temperatures. The efficacy of charging electric vehicles in cold climates and throughout the winter is substantially hampered by this issue.
A team of researchers from the Argonne and Lawrence Berkeley national labs created a fluorinated electrolyte that functions effectively even in sub-zero temperatures. “Our research thus demonstrated how to tailor the atomic structure of electrolyte solvents to design new electrolytes for sub-zero temperatures,” says John Zhang, who headed the research team at Argonne National Lab.
According to Zhang, a senior chemist and group leader in Argonne’s Chemical Sciences and Engineering division, “Our team not only found an antifreeze electrolyte whose charging performance does not decline at minus 4 degrees Fahrenheit, but we also discovered, at the atomic level, what makes it so effective.” This low-temperature electrolyte can be used for consumer devices like computers and phones, electric grids, and battery technology for automobiles.
The electrolyte-containing carbonate solutions start to freeze when the temperature lowers. Because the lithium ions are so closely linked inside the solvent clusters, it loses their capacity to transfer lithium ions to the anode during charging. Because of this, those ions need significantly more energy than they need at ambient temperature to break free from their clusters and enter the interface layer. According to the experts, finding a superior solvent that wouldn’t freeze was the answer to the problem of poor performance in cold weather.
Related Article: Lithium-ion Battery Cell Types, LFP, NMC Cells Explained
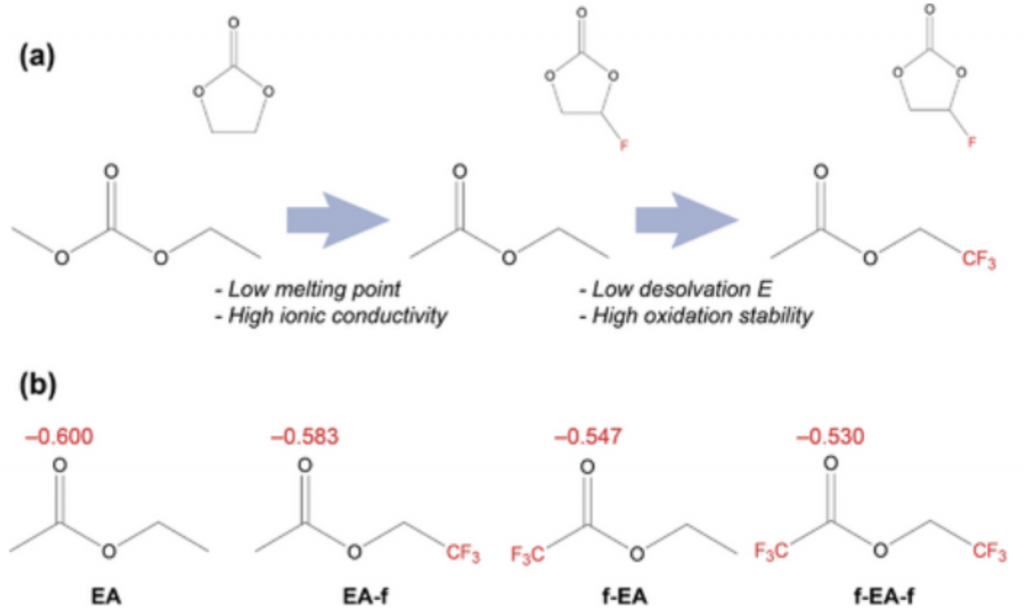
“One of the hardest issues for lithium-ion batteries is long-term cyclability at high C-rates and low temperatures. Scientists performed long-term cycling experiments under a variety of settings to demonstrate the superiority of our electrolytes.
“When a current of 2 C was supplied at 25 °C, the capacity retention of the fluorine-containing ethyl acetate electrolyte steadily decreased to 73% after 400 cycles, but the LiDFOB additive–containing electrolyte had the greatest capacity retention at 91% after 400 cycles. At a further high current of 6 C, this tendency is still present.
The electrolyte with the LiDFOB addition had the greatest capacity retention of 85% even after 500 cycles, whereas Gen 2 quickly declined to 34% within 50 cycles. Gen 2 and ethyl acetate electrolytes had a substantial capacity deterioration when a current of C/3 was supplied at 20 °C, equating to 7.5% and 34% capacity retention after 300 cycles, respectively.
“By comparison, the LiDFOB additive in the ethyl acetate with fluorine electrolyte showed a low capacity loss and sustained 97% capacity even after 300 cycles. Additionally, the EA-f electrolyte with LiDFOB additive demonstrated greater Coulombic efficiency than other electrolytes under all test circumstances. This cycle test result demonstrates electrolytes’ better stability for rapid charging and low-temperature operations.
_________________________________________________________________________________________________________________
The only thing we can reasonably be confident of is that the batteries in use in ten years will differ from those in use today in the same way that transistors differ from vacuum tubes. The EV revolution is only now beginning. We eagerly anticipate what will happen next.